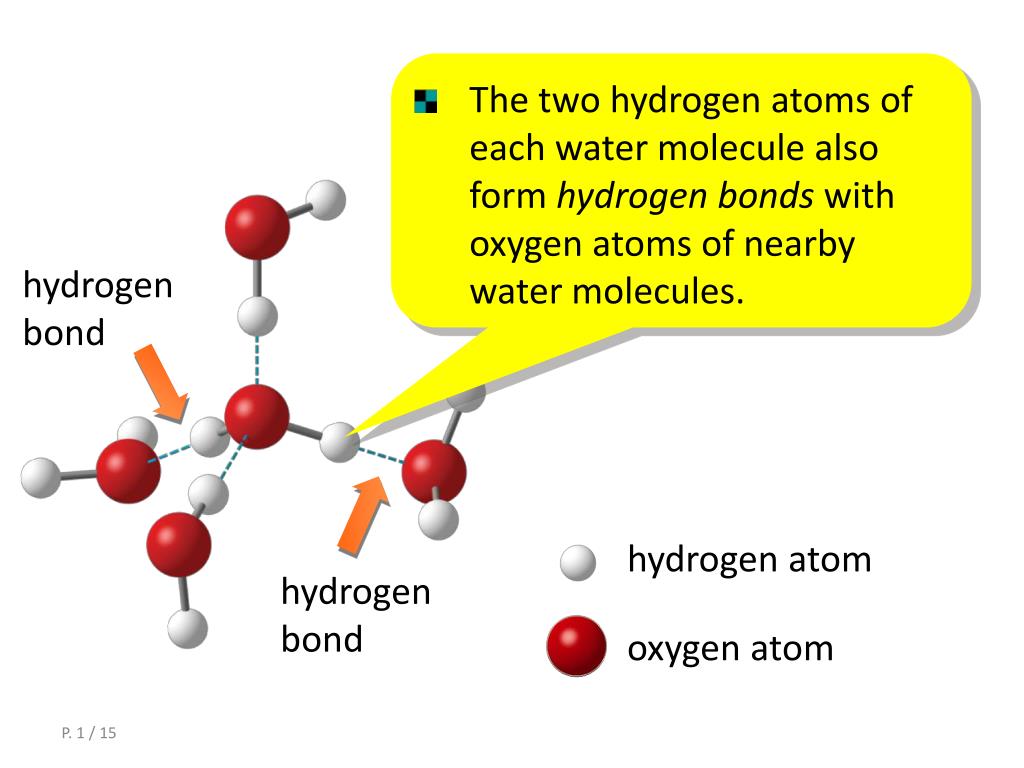
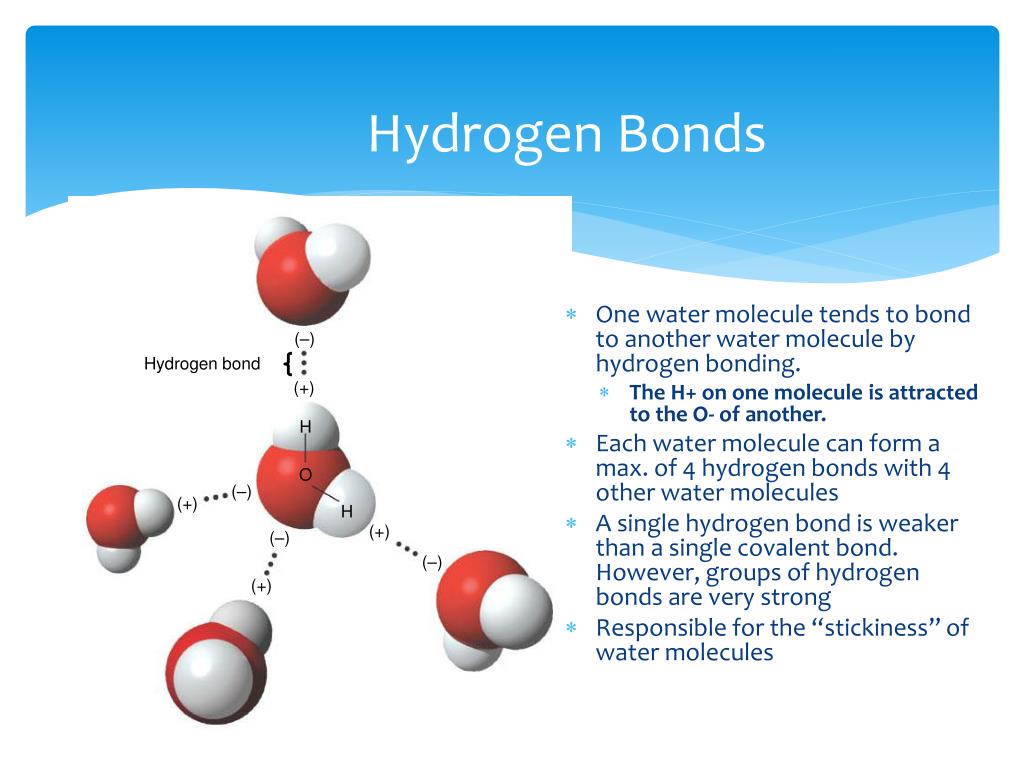
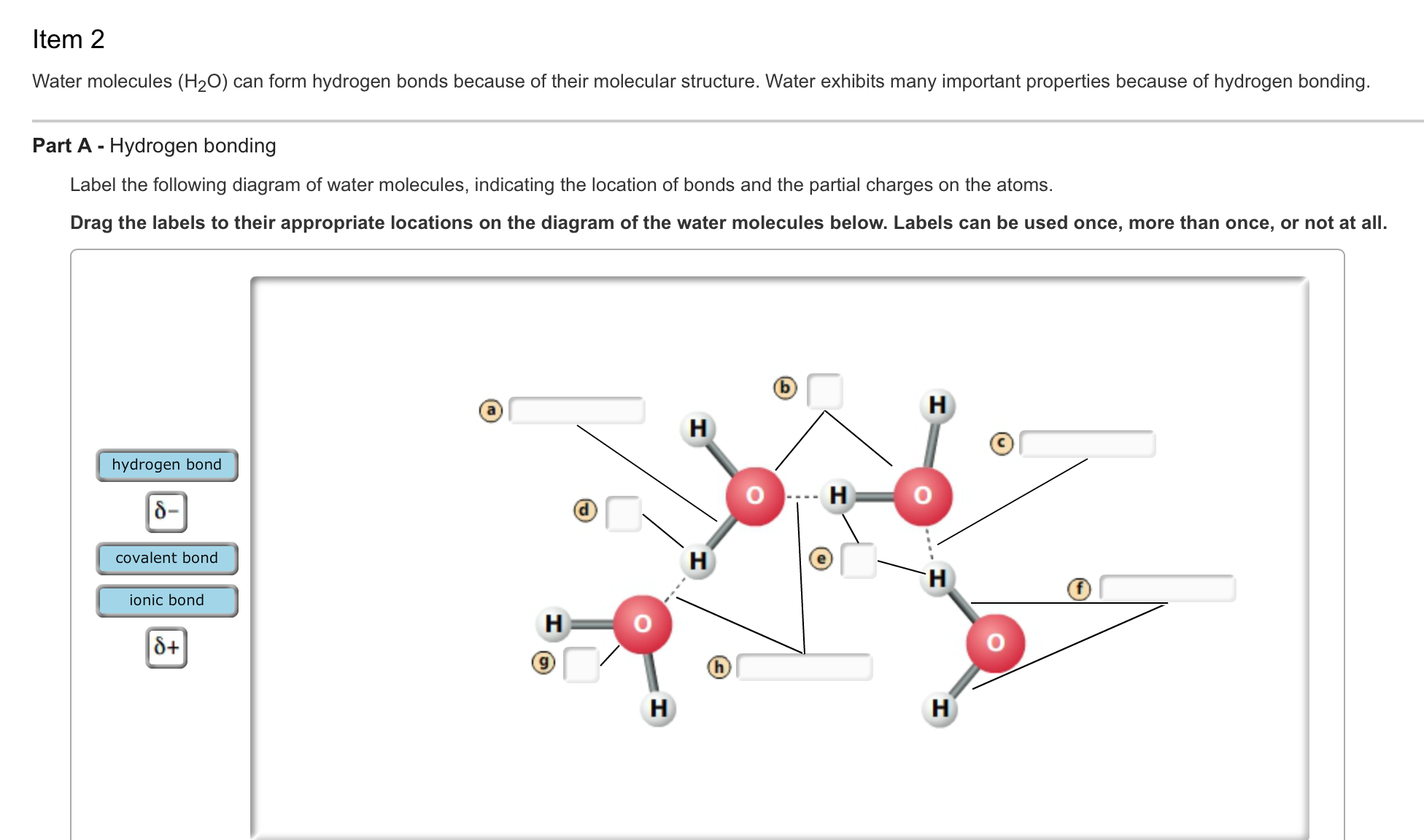
How Do Hydrogen Bonds Form Between Water Molecules
How Do Hydrogen Bonds Form Between Water Molecules - The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of.
Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule.
The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its.
Are Hydrogen Bonds The Strongest Interactions Between Molecules?
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules And Hydrogen Bonding Diagram
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules Hydrogen Bonds
The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules Hydrogen Bonds
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule.
Diagram of hydrogen bonding between two water molecules Download
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Draw A Hydrogen Bond Between Two Water Molecules
Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule. Identify three special properties of water that make it unusual for a molecule of its.
Water Molecules Hydrogen Bonds
Identify three special properties of water that make it unusual for a molecule of its. Hydrogen bonds form between water molecules when the partially positive end of. The covalent bonds of water make it a polar molecule.
hydrogen bond between water molecules Diagram Quizlet
Identify three special properties of water that make it unusual for a molecule of its. The covalent bonds of water make it a polar molecule. Hydrogen bonds form between water molecules when the partially positive end of.
The Covalent Bonds Of Water Make It A Polar Molecule.
Hydrogen bonds form between water molecules when the partially positive end of. Identify three special properties of water that make it unusual for a molecule of its.